Description
(Sterile Injectable Electrolyte for Hypokalemia Correction and Electrolyte Balance)
Clinical-Grade Potassium Supplementation for IV Use in Electrolyte Management
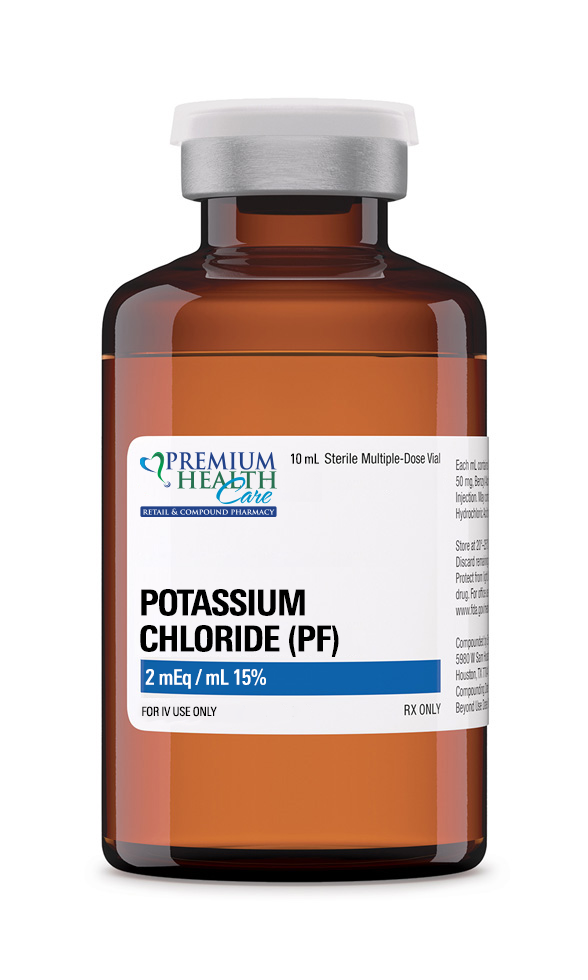
Potassium Chloride (KCl) 2 mEq/mL (15%) – 10 mL Preservative-Free Vial is a sterile, high-concentration injectable solution intended for intravenous electrolyte replacement therapy under medical supervision. This formulation is designed to treat or prevent hypokalemia (low serum potassium levels) in patients who are unable to consume adequate potassium orally or who are experiencing rapid potassium depletion due to illness, medication, or fluid loss.
This preservative-free solution is compounded under USP <797> sterile conditions and is suitable for hospital, ambulatory infusion, critical care, and surgical recovery settings. It must always be diluted in a compatible IV fluid prior to administration and infused slowly to avoid serious cardiac or neurological complications.
Product Details
- Active Ingredient: Potassium Chloride
- Concentration: 2 mEq/mL (equivalent to 15% w/v)
- Total Volume: 10 mL
- Total Potassium Content: 20 mEq per vial
- Formulation: Sterile, preservative-free solution
- Administration: Intravenous infusion only, after dilution
- Storage: Refrigerate at 2–8°C or store at controlled room temperature; protect from light
- Compounded in compliance with USP <797> and USP <795> standards
Clinical Use and Indications
Potassium chloride is a vital mineral and electrolyte involved in a wide range of physiological processes. Injectable KCl is indicated for the treatment and prevention of potassium deficiency, particularly in acute or critically ill patients.
Indications:
- Hypokalemia, mild to severe
- Cardiac arrhythmias associated with low potassium
- Electrolyte repletion in post-surgical, trauma, or dehydrated patients
- Potassium loss due to vomiting, diarrhea, or diuretic use
- Parenteral nutrition protocols
This formulation is particularly beneficial for patients with:
- Impaired oral intake
- Malabsorption syndromes
- Ongoing gastrointestinal losses
- Potassium-wasting medications (e.g., loop diuretics, corticosteroids, amphotericin B)
Mechanism of Action
Potassium plays a central role in:
- Cellular membrane potential and nerve impulse conduction
- Cardiac muscle contraction and rhythm regulation
- Skeletal muscle function
- Acid-base balance and fluid regulation
- Enzyme activation in metabolic pathways
Correcting hypokalemia restores normal intracellular and extracellular potassium distribution, thereby stabilizing cardiac and neuromuscular function and preventing life-threatening arrhythmias.
Administration Guidelines
- Route: Intravenous only, and must be diluted prior to administration
- Dilution: Typically mixed in 100–1,000 mL of a compatible IV solution (e.g., 0.9% sodium chloride or D5W)
- Infusion Rate:
- Peripheral line: typically ≤10 mEq/hour
- Central line: may be infused at up to 20 mEq/hour under close monitoring
- Monitoring: Continuous ECG monitoring is required for rapid or high-dose infusions. Frequent lab evaluation of serum potassium, renal function, and acid-base status is essential
Warnings and Precautions
DO NOT ADMINISTER UNDILUTED. Rapid infusion or administration of concentrated potassium chloride can cause hyperkalemia, cardiac arrest, and death.
Contraindications:
- Hyperkalemia or conditions associated with elevated potassium (e.g., renal failure)
- Untreated Addison’s disease
- Severe tissue trauma or burns in early stages
- Use of potassium-sparing diuretics (unless closely monitored)
Precautions:
- Monitor serum potassium, renal function, and urine output regularly
- Use with caution in patients with renal impairment or cardiac conduction disorders
Adverse Reactions
When used appropriately, potassium chloride is well tolerated. However, adverse effects may include:
- Pain or irritation at the injection site (especially in peripheral veins)
- Phlebitis or thrombosis
- Hyperkalemia, with symptoms such as bradycardia, ECG changes, weakness, and paresthesia
- Nausea or gastrointestinal upset when given in high doses
Storage and Handling
- Store refrigerated at 2–8°C, or at controlled room temperature (15–30°C)
- Protect from light and freezing
- Discard vial 28 days after first puncture if not used entirely
- Use aseptic technique for every withdrawal
Intended Use
- Hospital infusion and emergency care
- Outpatient IV therapy clinics
- Parenteral nutrition protocols
- Post-operative electrolyte balance
- Functional or integrative therapy requiring potassium repletion
Potassium Chloride Injection 2 mEq/mL (15%) – 10 mL provides clinicians with a sterile, concentrated, and preservative-free option for managing moderate to severe hypokalemia and maintaining critical electrolyte balance. When properly diluted and monitored, it ensures effective and safe potassium supplementation in high-risk or deficient patients.





Reviews
There are no reviews yet.