Description
(Sterile Injectable Electrolyte for Neuromuscular, Cardiac, and Metabolic Support)
High-Concentration Magnesium Sulfate for Critical Care and Functional Wellness
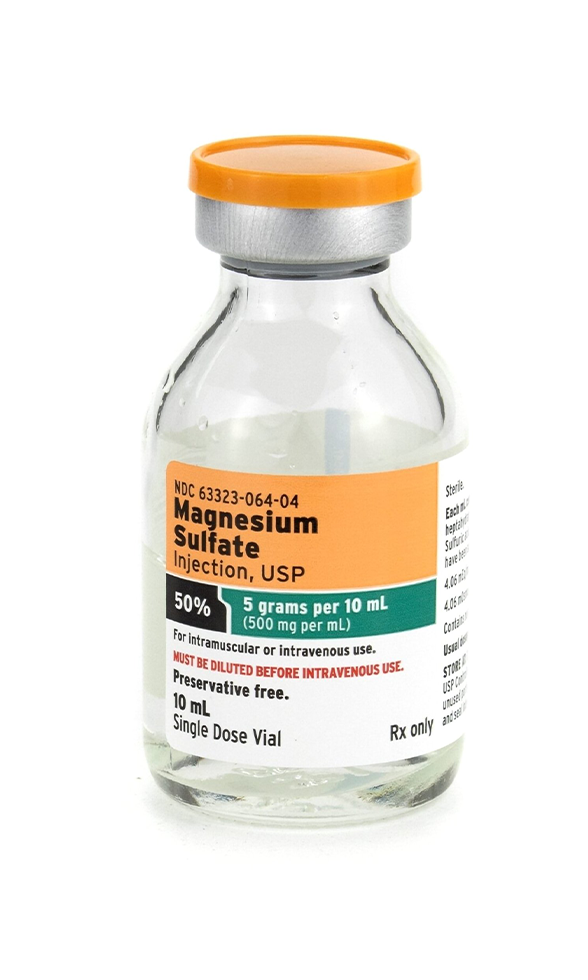
Magnesium Sulfate Injection 50% – 500 mg/mL, 10 mL is a sterile, preservative-free injectable formulation of Magnesium Sulfate Heptahydrate, compounded for use in acute, preventive, and supportive therapies. Designed for intravenous (IV) or intramuscular (IM) administration under licensed medical supervision, it plays a vital role in restoring and maintaining intracellular magnesium balance.
Magnesium is a critical cofactor in over 300 enzymatic reactions. It is essential for cardiac rhythm stabilization, neuromuscular transmission, vascular tone regulation, and energy production. Magnesium Sulfate is frequently used in both emergency settings (e.g., seizures, arrhythmias, eclampsia) and functional medicine protocols for stress modulation, migraine relief, and metabolic optimization.
Product Composition
- Active Ingredient: Magnesium Sulfate (as Heptahydrate)
- Concentration: 500 mg/mL
- Total Volume: 10 mL
- Formulation: Preservative-free, sterile solution
- Osmolarity: Hypertonic; for dilution before IV use
- Route of Administration: IM or IV (diluted)
- Compounded in a USP <797> sterile facility
- Packaging: Amber vial to protect from light exposure
Mechanism of Action
Magnesium acts as an intracellular cation influencing multiple physiological systems:
- Stabilizes excitable membranes by modulating calcium and potassium transport
- Inhibits neuromuscular transmission by interfering with acetylcholine release
- Promotes vasodilation via calcium channel blocking
- Acts as an antiarrhythmic in ventricular arrhythmias
- Supports ATP production, glucose regulation, and mitochondrial function
- Reduces NMDA receptor overactivation to mitigate excitotoxicity and pain
Key Clinical Applications
1. Electrolyte Repletion
Used in patients with:
- Hypomagnesemia due to diuretics, alcoholism, GI loss
- Refeeding syndrome or prolonged parenteral nutrition
- Magnesium-wasting syndromes
2. Cardiovascular Support
Administered in:
- Torsades de Pointes and refractory ventricular arrhythmias
- Digitalis toxicity
- Hypertension in pregnancy
- Vasospastic angina and arrhythmia prevention
3. Neuromuscular Stabilization
Magnesium sulfate is used for:
- Preeclampsia and eclampsia seizure prophylaxis
- Acute asthma (as bronchodilator adjunct)
- Migraine prophylaxis and relief
- Muscle cramps, spasms, or fibromyalgia
4. IV Therapy and Functional Medicine
Commonly incorporated into IV nutrient drips (e.g., Myers’ Cocktail) to:
- Relieve chronic fatigue and tension
- Improve sleep quality and stress resilience
- Support energy metabolism
- Reduce PMS or menstrual-related symptoms
Dosage and Administration
- IM Use: Undiluted or slightly diluted, 1–5 mL in gluteal muscle
- IV Use: Always dilute in compatible carrier fluid (e.g., normal saline or D5W)
- Typical IV dose: 1–2 grams (2–4 mL) over 20–60 minutes
- Acute protocols (e.g., eclampsia): Up to 4–6 grams bolus followed by maintenance drip
- Rate and volume must be customized based on renal function, severity of deficiency, and clinical setting
Safety and Monitoring
Monitoring is essential, particularly for IV administration, to avoid toxicity or electrolyte imbalances.
- Common side effects (dose-dependent):
- Flushing
- Hypotension
- Diaphoresis
- Injection site discomfort (IM)
- Serious adverse effects (usually with overdose):
- Respiratory depression
- Bradycardia
- Loss of deep tendon reflexes
- Cardiac arrest (rare)
Serum magnesium levels, deep tendon reflexes, respiratory rate, and urine output should be monitored in high-dose or continuous infusions.
Contraindications and Cautions
- Severe renal impairment without dialysis access
- Myasthenia gravis (may worsen muscle weakness)
- Concurrent use with calcium channel blockers may increase hypotensive risk
- Use caution in elderly, pediatric, or pregnant patients unless clinically indicated
Antidote for toxicity: Calcium gluconate, IV slow push
Storage and Handling
- Store at 20–25°C (68–77°F)
- Do not freeze
- Protect from light
- Use sterile technique; discard within 28 days after first use
Clinical Use Settings
Magnesium Sulfate 50% is ideal for:
- Emergency departments and ICUs
- Obstetrics and gynecology clinics
- Cardiology and electrophysiology units
- Pain and migraine centers
- Functional and integrative medicine practices
- IV nutrient and recovery therapy clinics
Magnesium Sulfate 500 mg/mL – 10 mL Preservative-Free Injectable provides a critical, high-concentration magnesium source for targeted correction of deficiency, stabilization of neuromuscular and cardiovascular function, and broad metabolic support. Whether for acute care or functional wellness, this formulation remains a foundational therapeutic in evidence-based clinical protocols.



Reviews
There are no reviews yet.