Description
(High-Concentration Parenteral Magnesium for Acute Deficiency and Therapeutic Use)
Concentrated Intravenous Magnesium for Critical Electrolyte Replenishment and Therapeutic Intervention
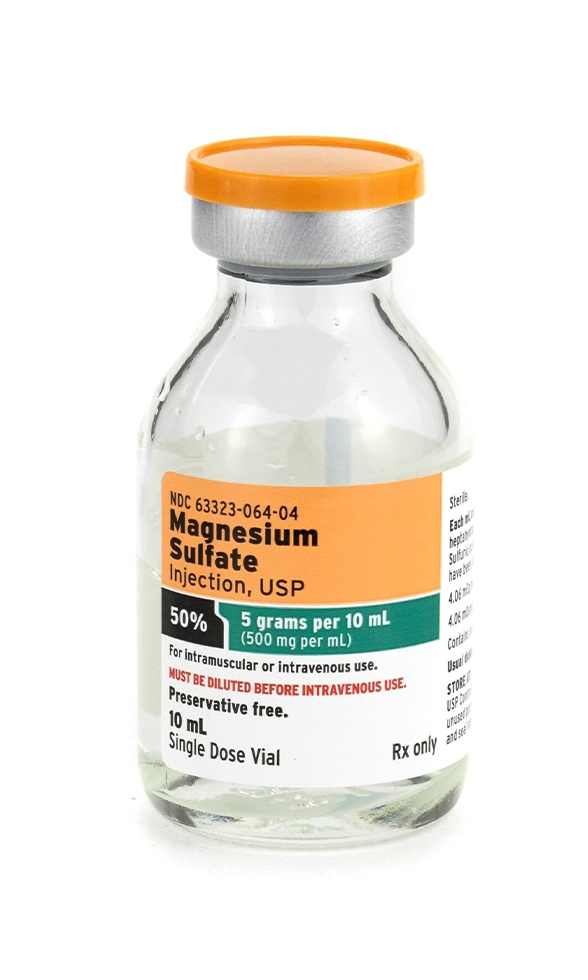
Magnesium Sulfate Injection 50% (500 mg/mL) – 10 mL is a sterile, preservative-free injectable solution formulated for intravenous (IV) or intramuscular (IM) use. This high-concentration preparation delivers 5 grams of magnesium sulfate per 10 mL vial, making it highly effective for acute magnesium repletion, obstetric emergencies, cardiac arrhythmia management, and neuromuscular stabilization.
Magnesium sulfate is a critical mineral and electrolyte essential for numerous physiological processes, including muscle and nerve function, cardiac rhythm stability, vasodilation, and enzyme activity. This formulation is intended for use by qualified medical professionals in emergency rooms, ICUs, obstetric units, and specialty infusion settings.
Product Specifications
- Active Ingredient: Magnesium sulfate heptahydrate
- Concentration: 50% solution (500 mg/mL)
- Total Volume: 10 mL per vial (5,000 mg total magnesium sulfate)
- Formulation: Aqueous, sterile, preservative-free
- Administration Routes: IV infusion, IV push (diluted), IM injection
- Osmolarity: Hypertonic; must be diluted for IV use
- Storage: Store at controlled room temperature (20–25°C); protect from freezing
- Packaging: Single-dose vial; discard unused portion
Pharmacology and Mechanism of Action
Magnesium plays a vital role in cellular physiology. As a cofactor in over 300 enzymatic reactions, magnesium regulates neuromuscular transmission, myocardial excitability, and vascular tone.
Magnesium sulfate acts by:
- Slowing neuromuscular conduction
- Suppressing acetylcholine release
- Stabilizing cell membranes
- Inhibiting smooth muscle contraction
- Promoting vasodilation and anti-arrhythmic effects
These properties make it essential for treating hypomagnesemia, eclampsia, torsades de pointes, and asthma exacerbations.
Clinical Indications
Magnesium Sulfate 50% Injection is indicated for:
- Acute symptomatic hypomagnesemia
- Seizure prophylaxis and treatment in preeclampsia and eclampsia
- Torsades de pointes and other ventricular arrhythmias associated with low magnesium
- Adjunctive treatment in severe asthma exacerbations (as bronchodilator)
- Neuroprotection in premature labor (neurodevelopmental outcomes in neonates)
- Nutritional support in TPN protocols
Dosage and Administration
Important: Due to its hypertonicity, the 50% solution must always be diluted prior to IV administration.
Standard Adult Dosing (based on indication):
- Hypomagnesemia: 1–2 g diluted in 50–100 mL normal saline, infused over 1–2 hours
- Eclampsia: Loading dose of 4–6 g IV over 20–30 minutes, then maintenance of 1–2 g/hour
- Torsades de pointes: 1–2 g IV push over 15–20 minutes
- Severe asthma: 1–2 g IV over 20 minutes as adjunct therapy
- IM administration: 1–5 g deep IM per gluteal muscle, may be painful—divide large doses
Maximum safe dosage depends on renal function, patient status, and clinical setting. Continuous monitoring of magnesium levels and vital signs is essential during high-dose administration.
Safety and Monitoring
Common side effects (typically dose-related):
- Flushing and warmth
- Hypotension
- Muscle weakness
- Drowsiness
- Injection site irritation or pain
Serious adverse effects (especially in overdose):
- Respiratory depression
- Bradycardia
- Complete heart block
- Loss of deep tendon reflexes
- Cardiac arrest
Calcium gluconate is the antidote for magnesium toxicity and should be readily available during high-dose or continuous magnesium therapy.
Warnings and Precautions
- Renal impairment: Reduce dose and monitor closely to prevent accumulation
- Pregnancy: Used therapeutically in obstetrics, but prolonged or high-dose use should be monitored for fetal effects
- Neonates: Caution in maternal dosing near delivery
- Concomitant medications: Use caution with neuromuscular blockers, CNS depressants, or other electrolytes
Monitoring parameters:
- Serum magnesium (target range: 1.7–2.2 mg/dL; higher in seizure prophylaxis)
- Respiratory rate, urine output, blood pressure, reflexes
- ECG monitoring if used for cardiac rhythm stabilization
Storage and Handling
- Store at 20–25°C (68–77°F)
- Do not freeze
- Protect from light
- Use aseptic technique when handling
- Single-use vial; discard unused portion after opening
Ideal for Use In:
- Critical care and ICU settings
- Labor and delivery units
- Emergency departments
- Cardiology and electrophysiology labs
- Pulmonology clinics and infusion centers
Magnesium Sulfate Injection 50% (500 mg/mL) – 10 mL Vial provides a powerful, reliable option for managing acute magnesium deficiency, obstetric emergencies, cardiac arrhythmias, and airway hyperresponsiveness. With its high concentration and fast onset of action, it remains an essential agent in emergency and inpatient medicine protocols. Proper dosing, dilution, and monitoring are critical to ensure therapeutic success and patient safety.



Reviews
There are no reviews yet.