Description
(Sterile Injectable Iron for the Treatment of Iron Deficiency Anemia)
Clinically Proven Intravenous Iron Therapy for Iron Deficiency Anemia
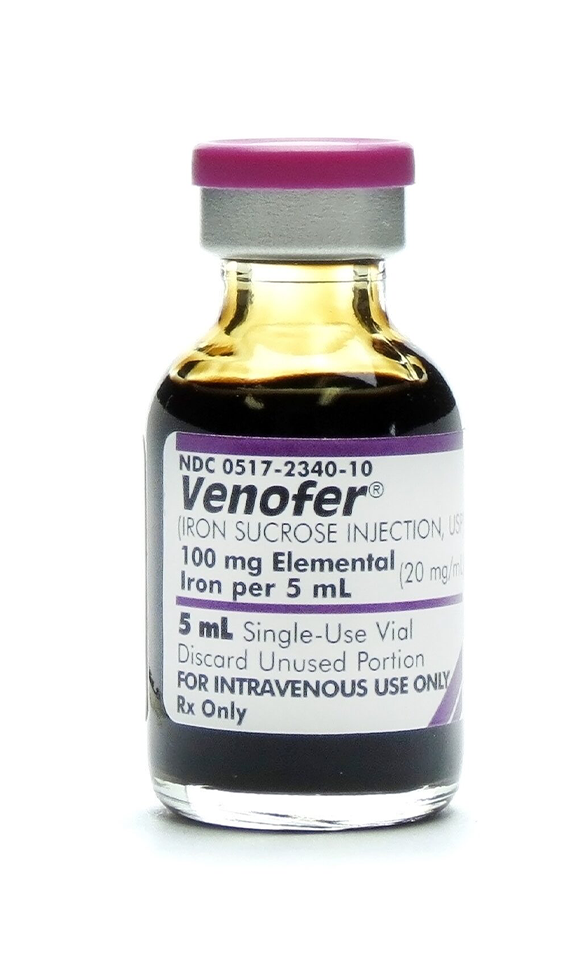
Iron Sucrose Injection (Venofer) 20 mg/mL – 5 mL vial is a sterile, preservative-free parenteral iron formulation indicated for the treatment of iron deficiency anemia (IDA), particularly in patients for whom oral iron therapy is ineffective, intolerable, or contraindicated. This formulation delivers 100 mg of elemental iron per vial and is intended for intravenous (IV) administration only.
Iron sucrose is a widely used, well-tolerated IV iron preparation known for its high safety profile, favorable tolerability, and low risk of hypersensitivity compared to older iron dextran products. It is frequently used in chronic kidney disease (CKD) patients (both dialysis-dependent and non-dialysis), as well as in cases of postpartum anemia, gastrointestinal malabsorption syndromes, or preoperative anemia correction.
Product Specifications
- Active Ingredient: Iron Sucrose Complex
- Concentration: 20 mg/mL
- Total Volume: 5 mL per vial
- Total Iron Content: 100 mg elemental iron
- Administration Route: Intravenous (IV) only
- Formulation: Aqueous solution, non-dextran based
- Preservative-Free, Latex-Free, Pyrogen-Free
- Storage: Store at 20–25°C (68–77°F); protect from light; do not freeze
- Manufacturer Equivalent: Venofer® (reference listed drug)
Mechanism of Action
Iron sucrose restores depleted iron stores by delivering iron directly into the bloodstream, where it is taken up by the reticuloendothelial system and transferred to transferrin for hemoglobin synthesis. Once incorporated into transferrin, iron is delivered to the bone marrow for red blood cell production.
Because it bypasses gastrointestinal absorption, it is ideal for rapid iron replenishment, especially in patients with absorption issues, chronic inflammation, or significant anemia requiring quick correction.
Indications
Iron Sucrose Injection is indicated for:
- Iron deficiency anemia in chronic kidney disease (CKD) – both dialysis-dependent and non-dialysis
- Iron deficiency anemia due to malabsorption syndromes (e.g., celiac disease, bariatric surgery)
- Intolerance or unresponsiveness to oral iron supplements
- Anemia in pregnancy/postpartum (under physician guidance)
- Pre-operative iron repletion for elective surgery patients
- Heavy menstrual bleeding leading to iron depletion
Dosage and Administration
- Each 5 mL vial contains 100 mg of elemental iron
- Administer IV slowly as undiluted injection over 2–5 minutes OR diluted in 100 mL 0.9% saline and infused over 15–60 minutes
- Typical adult dose: 100–200 mg per session; total cumulative doses depend on severity of anemia and iron stores
- CKD (hemodialysis): Often given during dialysis sessions 1–3 times per week
- Maximum single dose: Should not exceed 200 mg in a single infusion
Always use sterile technique. Do not administer intramuscularly or subcutaneously. Observe patient during and after infusion for adverse reactions.
Safety and Tolerability
Iron sucrose is generally well tolerated with a lower incidence of serious hypersensitivity reactions compared to older formulations like iron dextran.
Common adverse effects:
- Mild hypotension
- Nausea, vomiting
- Headache or dizziness
- Injection site burning or discoloration
- Muscle cramps
Rare but serious reactions:
- Anaphylactoid reactions (extremely rare)
- Iron overload with chronic excessive dosing
- Hypotension with rapid infusion
Monitoring Recommendations:
- Monitor hemoglobin, hematocrit, ferritin, and transferrin saturation regularly
- Avoid concurrent use with oral iron therapy
- Discontinue therapy once iron stores are replenished to prevent overload
Contraindications and Precautions
Contraindications:
- Known hypersensitivity to iron sucrose
- Evidence of iron overload (elevated ferritin or transferrin saturation)
- Anemia not caused by iron deficiency (e.g., hemolytic anemia)
Caution:
- Use cautiously in patients with liver disease, chronic inflammatory conditions, or multiple drug allergies
- Avoid during first trimester of pregnancy unless clearly needed
Storage and Handling
- Store at 20–25°C (68–77°F)
- Do not freeze
- Protect from light
- Do not use if solution is discolored or contains particulate matter
- Single-use vial – discard unused portion after opening
Ideal For Use In:
- Dialysis clinics and nephrology practices
- Hematology/oncology centers
- Women’s health and obstetrics (for postpartum anemia)
- Gastroenterology and bariatric surgery practices
- Primary care and infusion centers
Iron Sucrose Injection 20 mg/mL – 5 mL Vial (Venofer Equivalent) provides a reliable, fast-acting, and well-tolerated solution for iron deficiency anemia, especially when oral supplementation is ineffective. With a strong safety profile and extensive clinical usage in renal, GI, and gynecologic populations, it remains a cornerstone in modern IV iron replacement therapy.



Reviews
There are no reviews yet.